Antibiotic ‘Velcro’ Reveals New Mechanism to Trap Bacteria
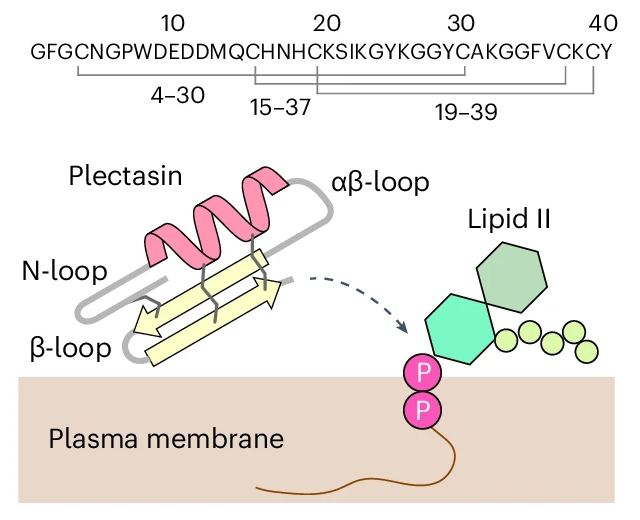
A team led by Utrecht University chemists has uncovered how the antibiotic plectasin fights bacteria through a unique ‘Velcro’ mechanism. Rather than simply binding to the bacterial cell wall precursor Lipid II, plectasin assembles into dense, supramolecular structures that trap the target on the bacterial membrane, making escape nearly impossible. This trapping action is further enhanced by calcium ions, which boost plectasin’s antibacterial activity. Published in Nature Microbiology, the findings highlight a new, self-assembling strategy in antibiotic action and may guide future drug design to combat antimicrobial resistance.
Publication:
Host defense peptide plectasin targets bacterial cell wall precursor Lipid II by a calcium-sensitive supramolecular mechanism
Shehrazade Jekhmane, Maik G.N. Derks, Sourav Maity, Cornelis J. Slingerland, Kamaleddin H. M. E. Tehrani, João Medeiros-Silva, Vicky Charitou, Danique Ammerlaan, Céline Fetz, Naomi A. Consoli, Eilidh J. Matheson, Rachel V. K. Cochrane, Mick van der Weijde, Barend O.W. Elenbaas, Francesca Lavore, Ruud Cox, Joseph H.F.F Lorent, Marc Baldus, Markus Künzler, Moreno Lelli, Stephen Cochrane, Nathaniel I. Martin, Wouter H. Roos, Eefjan Breukink, Markus Weingarth
Nature Microbiology 9, 1778–1791 (2024). doi:10.1038/s41564-024-01696-9